Hard Water Problems
What is Hard Water?
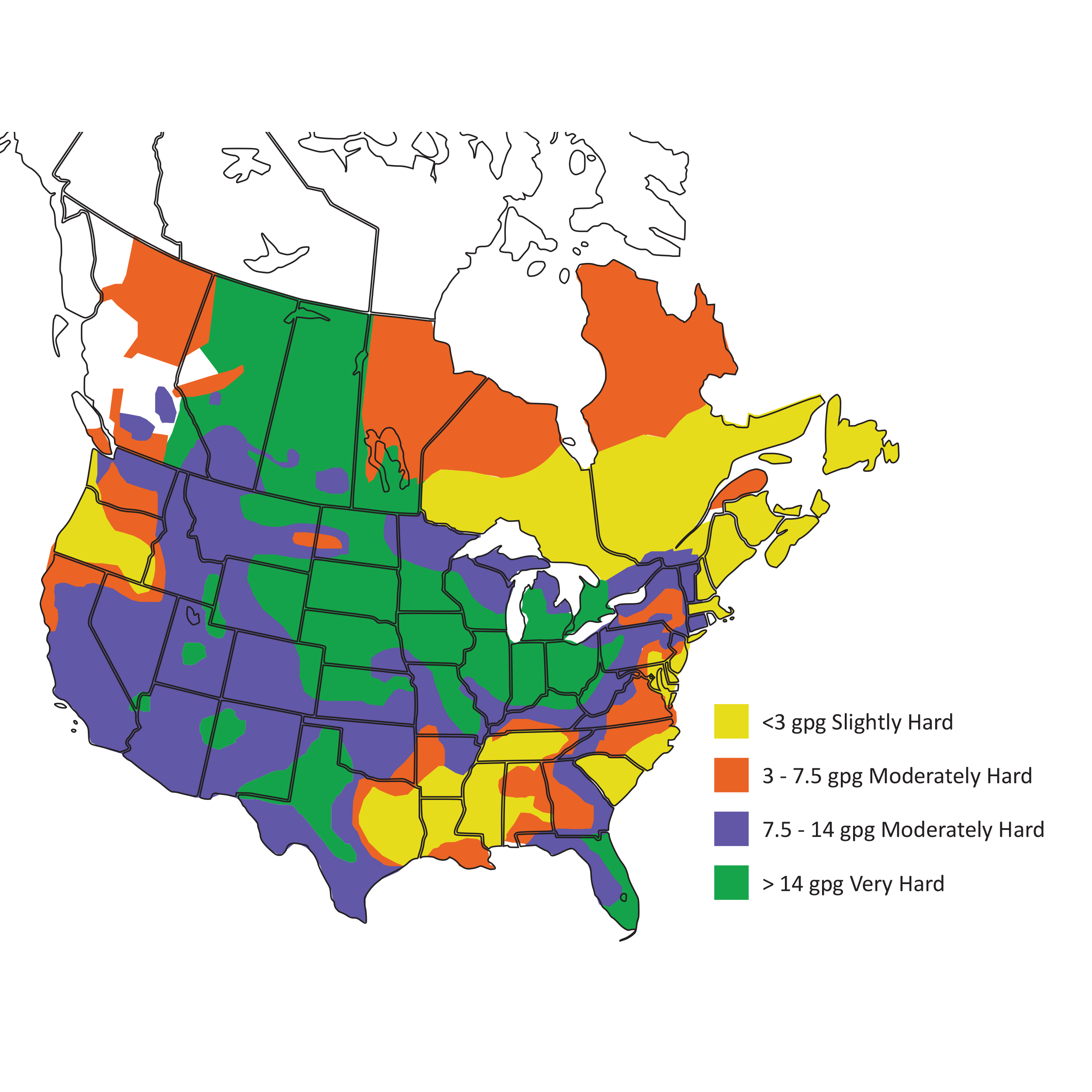
Hard water is caused by dissolved minerals calcium and magnesium and is measured as grains per gallon (gpg).
SOFT - 0.6 to 3.6 gpg
MODERATE - 3.6 to 7.0 gpg
HARD - 7.0 to 10.5 gpg
VERY HARD - > 10.5 gpg
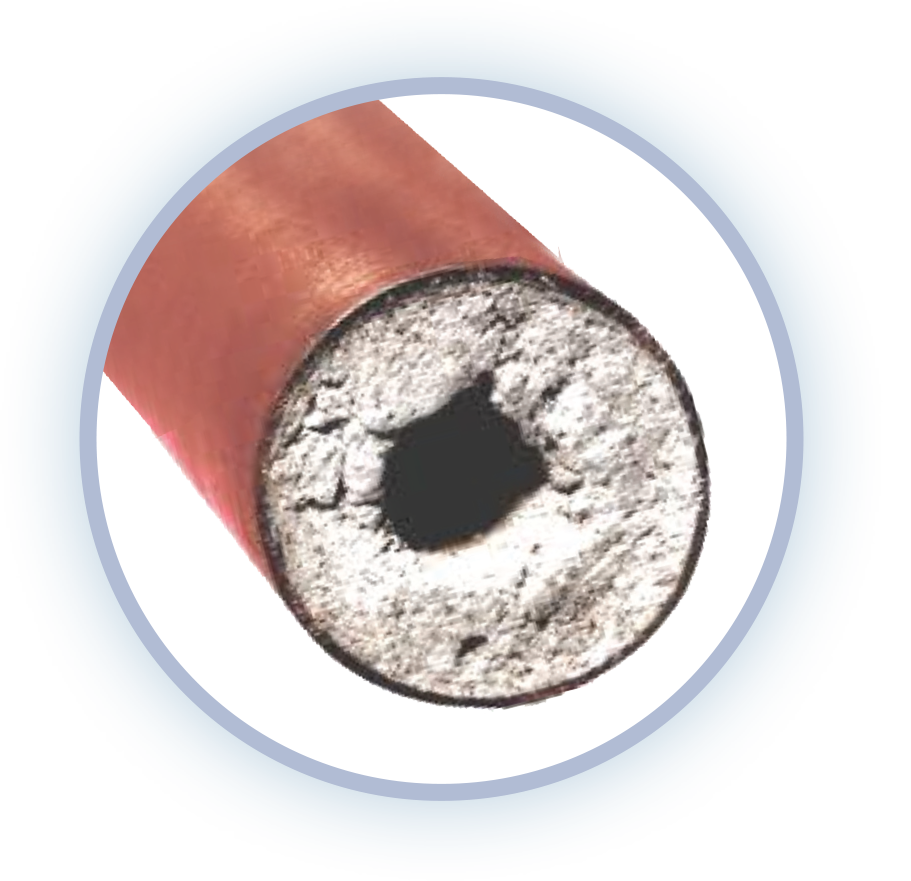
When combined with heat or soap hard water becomes extremely problematic. Heat and hard water create the formation of scale or rock. Scale builds up in plumbing, water using appliances, water heaters and fixtures. Soap reacts with hard water to create soap scum or curd making cleaning harder, your laundry duller and clinging to hair & skin.
Hard Water Facts:
Showerheads on hard water lost 75% of flow rate in less than 18 months
Tankless water heaters failed after only 1.6 years on hard water. On soft water they maintained original factory rating. Operating costs were also 34% less on 20 gpg water.
Gas water heaters are up to 24% more cost efficient on soft water
Electric water heaters can accumulate as much as 30 lbs of rock shortening the life of the heating element
You can use 50% less soap and detergent with soft water. Hard water makes soap work harder.
2009 Battelle Study, Water Quality Research Foundation
Check out this interesting article by the Water Quality Research Foundation about the benefits of softening hard water